Mycoplasma synoviae en gallinas ponedoras
Eficacia terapéutica de la tylvalosina (tvn) en aves positivas a mycoplasma synoviae, un enfoque del efecto clinico sobre la calidad de huevo (síndrome EAA) en la ponedora comercial
La infecciones por Mycoplasma synoviae (MS) se han incrementado en los últimos años, el MS es responsable de causar de traqueítis, aerosaculitis, sinovitis y EAA (Roturas en el ápice de la cascara) generando alteraciones en la calidad de huevo en un promedio de 12,7% y una disminución en la producción del 24%, a partir del dia 7 post infección. El objetivo de esta Revisión Sistemática es evaluar la potencia antibióticos macrolidos: Tylvalosina , Tylosina, Tilmicosina frente a desafíos de MS. Se identificaron 29 articulos publicados entre los años 2015-2023 de los cuales se seleccionaron 6 que cumplen con los requisitos científicos para realizar la evaluación estadística con una amplia variación de cepas de MS, en 5 zonas del mundo, el parámetro evaluado fue el MIC90. usando el programa estadístico Stargraphics centurión XVI prueba comparativa de medias por Tukey al 95% (P<0.05). En los resultados de 77 datos de lotes positivos a MS por cultivo comparados, la mayor potencia la tuvo la Tylvalosina 0.06336 mg/ml, la Tylosina con 0.41041mg/ml y la Tilmicosina 6.085mg/ml (P=0.0007), seguidamente realizamos una prueba de múltiples rangos para definir en que zona la Tylvalosina fue mas potente, se determino que Inglaterra tenia un MIC90 de 0.007mg/ml Vs China que fue de 0.135 MIC90, y para sur américa la sensibilidad fue de 0.063mg/ml (P=0.0097). Conclusión los anteriores datos recomiendan a la Tylvalosina como un antimicoplasmico potente que puede ayudar a controlar Mycoplasma synoviae.
Abstract
Infections due to Mycoplasma synoviae (MS) have increased in recent years. MS is responsible for causing tracheitis, air saculitis, synovitis and EAA (breaks in the apex of the shell), generating alterations in egg quality in an average of 12.7% and a decrease in production of 24%, starting on day 7 post infection. The objective of this Systematic Review is to evaluate the potency of macrolide antibiotics: Tylvalosin, Tylosin, Tilmicosin against MS challenges. 29 articles published between the years 2015-2023 were identified, of which 6 were selected that meet the scientific requirements to carry out the statistical evaluation with a wide variation of MS batches to MS by culture compared, the greatest strains, in 5 áreas of the world, the parameter evaluated was the MIC90. using the Stargraphics statistical program centurion XVI comparative test of means by Tukey at 95% (P<0.05). In the results of 77 data from positive potency was for Tylvalosin 0.06336 mg/ml, Tylosin with 0.41041 mg/ml and Tilmicosin 6.085 mg/ml (P=0.0007) Next we performed a multiple range test to define in which area Tylvalosin was more potent, it was determined that England had an MIC90 of 0.007mg/ml Vs China which was 0.135 MIC90, and for South America the sensitivity was 0.063mg/ml (P=0.0097). Conclusión: The above data recommend Tylvalosin as a potent antimycoplasmic that can help control Mycoplasma synoviae.
Mycoplasma synoviae
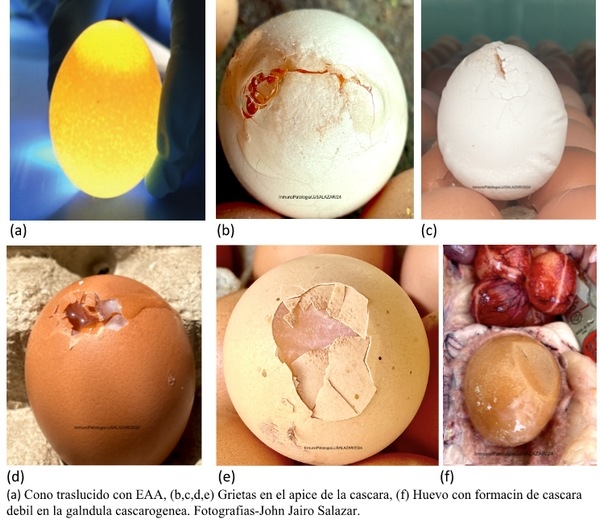
Tomado de (Kursa et al. 2019) https://doi.org/10.1186/s12917-018-1758-8
Materiales y Métodos
- Se debía informar el MIC (La Concentración Minima Inhibitoria) del comparativo entre los 3 macrolidos, Tylvalosina, Tylosina, Tilmicosina - Se debe informa el MIC 90% de los tres antibióticos macrolidos para realizar esl trabajo estadístico.
Extracción de datos
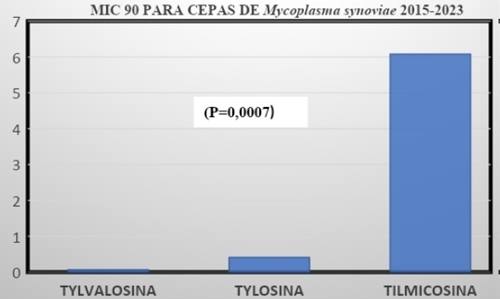
(P=0.0007) Diferencia altamente significativa
Zona | Casos | Medias-MIC 90 |
INGLATERRA | 1 | 0,007 |
AFRICA-EGIPTO | 1 | 0,015 |
ASIA-THAILANDIA | 6 | 0,015 |
EUROPA-CENTRAL | 1 | 0,025 |
SUR AMERICA-CERDA | 10 | 0,063 |
CHINA | 6 | 0,135 |
Conclusiones
El autor declara que no tiene conflicto de intereses.
[1] Molnár, S.; Szo ̋llo ̋si, L. Sustainability and Quality Aspects of Different Table Egg Production Systems: A Literature Review. Sustainability 2020, 12, 7884 https://doi.org/10.3390/su12197884 .
[2] Food and Agriculture Organization (FAO). FAOSTAT. Available online: http://www.fao.org/faostat/en/ (accessed on 4 February 2021).
[3] Programa Economico Fenavi-Fonav. Edicion Noviembre 30 del 2023. Numero 391.
[4] Feberwee, A., J. J. de Wit & W. J. M. Landman. Induction of eggshell apex abnormalities by Mycoplasma synoviae: field and experimental studies, Avian Pathology, 2009. 38:1, 77-85, DOI: 10.1080/03079450802662772 .
[5] Kleven, S.H. Mycoplasma synoviae infection. In Y.M. Saif, H.J. Barnes, J.R. Glisson, A.M. Fadly, L.R. McDougald & D.E. Swayne (Eds.), Diseases of Poultry, 2003. 11th edn (pp. 756-766). Ames: Iowa State Press.
[6] Landman, W.J.M. & Feberwee, A. Aerosol-induced Mycoplasma synoviae arthritis: the synergistic effect of infectious bronchitis virus infection. Avian Pathology, 2004. 33, 591-598.
[7] Morrow, C.J., Bell, I.G., Walker, S.B., Markham, P.F., Thorp, B.H. & Whithear, K.G. Isolation of Mycoplasma synoviae from infectious synovitis of chickens. Australian Veterinary Journal. 1990. 67, 121-12 .
[8] Giguere.S., Prescott.J., Patricia M.Antimicrobial Theraphy in veterinary medicine-Fifth edition.2013. 226
[9] Cerda ́, R G. I. Giacoboni, J. A. Xavier, P. L. Sansalone, and M. F. Landon. In Vitro Antibiotic Susceptibility of Field Isolates of Mycoplasma synoviae in Argentina Avian Disease .2002.46:215–218. DOI: 10.1637/0005-2086(2002)046[0215:IVASOF]2.0.CO;2.
[10] Royal GD. Results of genetic study of the Ms strain in the EAA case. Geographic diversity of Ms isolates based on MLST* of Ms isolates within and outside the Netherlands. 2023. info@gdanimalhealth.com www.gdanimalhealth.com .
[11] Ventura. C., Ramirez.G., Vera.V. Deteccion y Diferenciacion MycoplasmagallisepticumY Mycoplasmasynoviae Mediante la tecnica de PCR a partir de hisopos trauqeales en aves con sintomas respiratorios. 2012. Acta biol. Colomb., Vol. 17 n.º 3. 525-536.
[12] Mohammed HO, Carpenter TE, Yamamoto R. Economic impact of Mycoplasma gallisepticum and M. synoviae in commercial layer flocks. Avian Dis. 1987;31(3):477–82.
[13] Catania S, Bilato D, Gobbo F, Granato A, Terregino C, Iob L, Nicholas RAJ. Treatment of eggshell abnormalities and reduced egg production caused by Mycoplasma synoviae infection. Avian Dis. 2010;54(2):961–4.
[14] Kursa .O ., Anna Pakuła, Grzegorz Tomczyk, Sławomir Paśko and Anna Sawicka Eggshell apex abnormalities caused by two different Mycoplasma synoviae genotypes and evaluation of eggshell anomalies by full-field optical coherence tomography Veterinary Research. 2019 15:1 https://doi.org/10.1186/s12917-018-1758-8
[15] Eun-Ok Jeon, Jong-Nyeo Kim, Hae-Rim Lee, Bon-Sang Koo, Kyeong-Cheol Min, Moo-Sung Han, Seung-Baek Lee, Yeon-Ji Bae1, Jong-Suk Mo1, Sun-Hyung Cho1, Chang-Hee Lee, In-Pil Mo. Eggshell apex abnormalities associated with Mycoplasma synoviae infection in layers. J. Vet. Sci. 2014, 15(4), 579-582. http://dx.doi.org/10.4142/jvs.2014.15.4.579
[16] Fatma, I., Abo El-Ela, 2H., A. El-Banna, 3Manal., B. El- Deen, 1A.A. El-Gendy., and 1M.A. Tohamy . Pharma- cokinetics of Tylvalosin Alone or in Combination with Vitamin E in Broiler Chickens . 2015Asian Journal of Animal and Veterinary Advances 10 (10): 556-566.
[17] Ammar Haki Salman, S.A.H. Youssef, Amer Ramadan and Ahmed M. Soliman. Pharmacokinetics of tylvalosin in healthy and experimentally mycoplasma gallisepticum infect- ed broiler chickens. 2016 International Journal of PharmTech Re- search,9(10): 72-80.
[18] Salazar .J Efecto Farmacologico de la Tylvalosina (Pk/Pd) frente a desafios de Mycoplasma gallisepticum en pollos de engorde. 2022. Plumazos V75: 16-28.
[19] Limpavithayakul. K ., Jiroj Sasipreeyajan & Somsak Pakpinyo Molecular characterization and antimicrobial susceptibility profiles of Thai Mycoplasma synoviae isolates 2023. Nature. Scientific reports 13:2002 | https://doi.org/10.1038/s41598-023-29266-9 .
[20] Zsuzsa Kreizinger, Dénes Grózner, Kinga M. Sulyok, Kristin Nilsson, Veronika Hrivnák, Dušan Benčina and Miklós Gyuranec. Antibiotic susceptibility profiles of Mycoplasma synoviae strains originating from Central and Eastern Europe .2017 13:342 . DOI 10.1186/s12917-017-1266-2 .
[21] Wang, C., Naiji Zho, Haopeng Liu , Rongkun Yang, Weitao Cui, Qingrong Xu1, Yuncai Xiao, Sishun Hu, Rui Zhou, Zili Li and Zutao Zhou . Identification and antibiotic susceptibility evaluation of Mycoplasma synoviae isolated from chickens in central China 2022. Animal Diseases. 2:28 . https://doi.org/10.1186/s44149-022-00060-w .
[22] Esraa Fekry, Eman Abdeen , Youserya M. Hashem , Abdelaziz, E.E. , Alaa El Din H. Mostapha. Molecular Characterization and Antimicrobial Susceptibility of Mycoplasma Species Isolated from Broilers and Breeder Chickens .2023. AJVS. Vol. 79 (1): 17-23 Oct DOI: 10.5455/ajvs.148713 .
[23] Khaled Hussein. New insights into treating mycoplasma without compromising egg quality. International Poultry Production. 2015. Volume 24 Number 7 45 .
[24] Lockaby.S., Laureman.H., Kleven H.Pathogenecity of Mycoplasma synovia in Broilers chikens.1998. Veth Pathol 75:138-175.
[25] Slavec.B, Rebeka Lucijana Berčič, Ivanka Cizelj, Mojca Narat, Olga Zorman-Rojs, Peter Dovè, Dušan Benčina . Variation of vlhA gene in Mycoplasma synoviae clones isolated from chickens. 2011. pp.1. DOI 10.1080/03079457.2011.604840 .
[26] Frey, M. C., R. Hanson, and D. P. Anderson. A medium for the isolation of avian mycoplasmas. Am. J. 1968. Vet. Res. 29:2164–2171.
[27] Tsuchiya, M., K. Suzukake, M. Hori, T. Sawa, T. Takeuchi, and H. Umezawa. Studies on the effects of 3-acetyl-4-isovaleriltylosin against multiple-drug resistant strains of Staphylococcus aureus. J. Antibiot.1981. 34:305–312.
[28] Sumano.H., Gutierrez.L. Farmacologia Clinica en Aves. 2005 .ISBN: 970-94778-03 Pp 24-25.

Excelente reseña. Muchas gracias.
Pero, para los que trabajamos en el campo, y somos responsables de los residuos de medicamentos, en el producto final (huevos comerciales por ejemplo) pregunto:
1. La tylosina se detecta en la yema hasta por 7 días. ¿Qué hacer con el huevo durante este período?
2. ¿Cuál es el tiempo de retiro (withdrawal) de Tylvalosina y Tylmicosina para poder utilizar los huevos de mesa o comerciales?
3. ¿No sería más lógico y profesional invertir en bioseguridad, galpones monoedad y vacunación contra Mycoplasmas?
Gracias por la respuesta que realicen














