Meta análisis: Efecto Inmunomodulador sobre la IgA, generado por los ß-glucanos 1,3/1,6 (Sacharomices cerevisiae) en Pollos de engorde, desafiados con Salmonella entérica
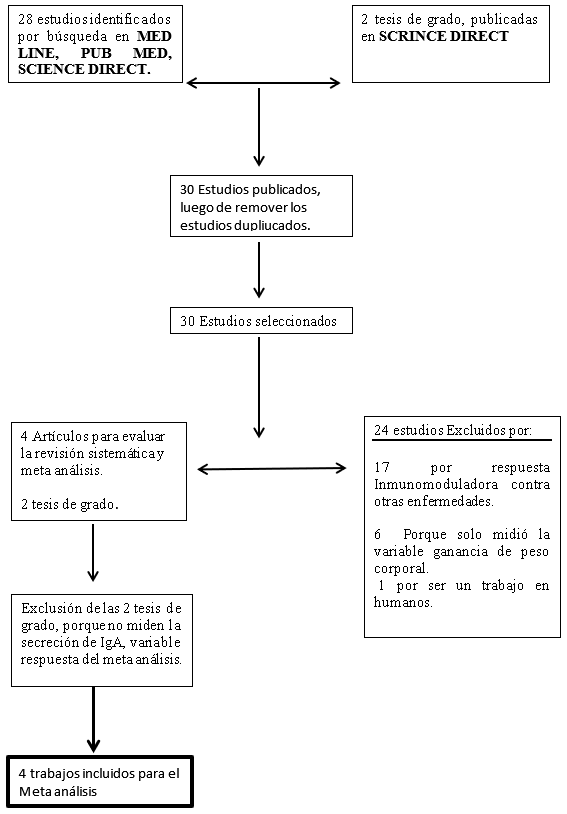
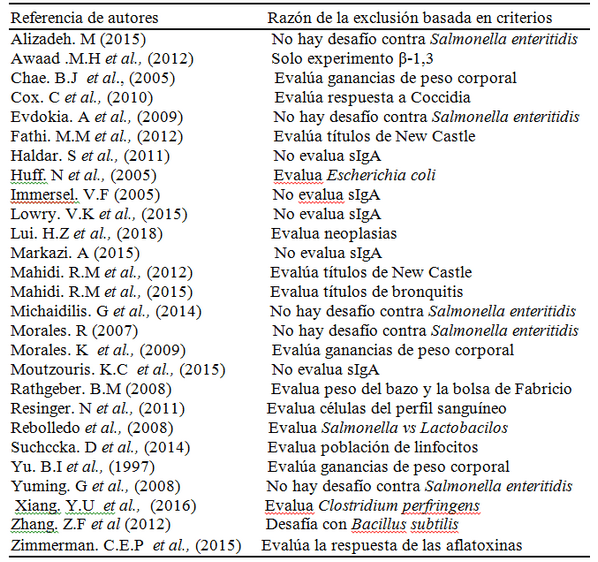
Este estudio investigo el efecto inmunomodulador de los β- glucanos 1,3/1,6 provenientes de la pared de levadura (Sacharomices cereviciae) y el efecto sobre la secreción de la Inmunoglobulina A (sIgA) en pollos desafiados con Salmonella enteritidis, se realizó un Meta análisis sobre la literatura publicada entre los años 2000 al 2018, utilizando las bases de datos computarizadas (PUB MED, MED LINE, SCIENCE DIRECT), sobre la calidad de los datos, se aplicó el programa QUALITY CONSORT, El proceso de revisión sistemática y meta análisis arrojo 30 estudios, de estos se eliminaron 26 trabajos de investigación que no cumplían con los requisitos , quedando 4 artículos, con un total de 356 broilers; El análisis de ANOVA de la gran media arroja que se presenta un aumento de la sIgA a una dosis de 25mg/k (P>0.4945) en comparación con los del grupo control que no fueron suplementados con β-glucanos pero desafiados con Salmonella enteritidis , demostrando una gran variación en la producción de IgA en las dosis que van desde 25-100mg/k; de igual marea al enfrentar dichas dosis y su efecto con el tiempo de uso, se observó que alos 15 días de uso continuo de los β-glucanos 1,3/1,6 la secreción de sIgA se mantenía estable; Nuestro resultados indican que la suplementación en la dieta con los β-glucanos 1,3/1,6 pueden incrementar la secreción de sIgA cuando se usa un dosis de 25mg/k por un periodo de 21 días en aves desafiadas con Salmonella enteritidis por esta razón pueden disminuir la colonización de la salmonella en el hígado en un 79%, reportado por el análisis de las medias poblacionales.
Palabras claves: β-glucanos 1,3/1,6, Sacharomices cereviciae, Salmonella, sIgA
This study investigated of immunomodulator effect of the β- 1,3/1,6 glucans coming of the cell wall yeast (Sacharomices cereviciae) and the effect on secretion of Immunoglobulin A(sIgA), in broilers challenged with Salmonella enteritidis, it has been made meta análisis on published literatura between years 2000 to the 2018, using computerized databases (PUB MED, MED LINE, SCIENCE DIRECT), on the quality of the data, the program was applied QUALITY CONSORT, The preces of the systematic review and meta analysis daring 30 papers, of these were eliminated 26 papers , leaving 4 papers with a total 356 broilers chikens; The ANOVA analysis of the great average show that there is an increase in sIgA a doses of the 25mg/k (P>0.4945); compared to those in the control group that were no supplemented with β- glucans but challenged with Salmonella enteritidis showing a great varition in the production of sIgA, the dosis between 25 – 100mg/k of equal tide when facing these doses in the effect on the over time it was observed after 15 days of continuios use of β- 1,3/1,6 glucans the sIgA it was mantein estable. Our results indicate wath dietary suplementation with β- 1,3/1,6 glucans at doses of 25mg/k by 21 day , increase if sedcfretion sIgA in broilers chikens challenged with Salmonella enteritidis, for this reason, can reduce colonization of Salmonella in the liver
Key words: β- 1,3/1,6 glucans, Sacharomices cereviciae, Salmonella, sIgA

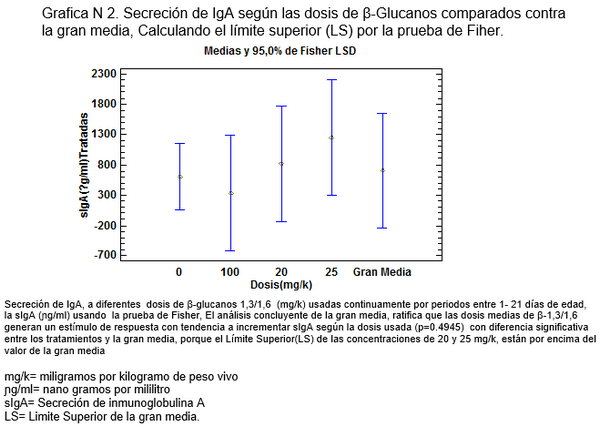
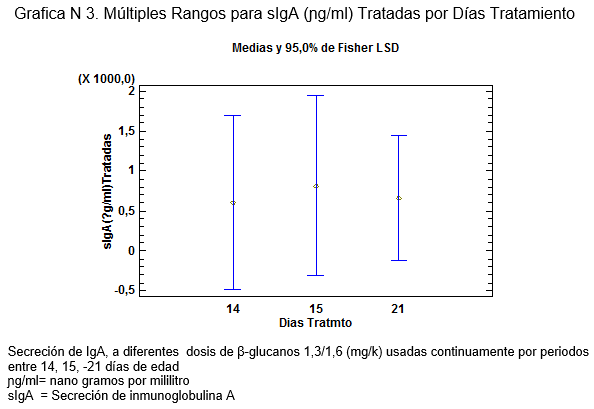
sIgA = Secreción de inmunoglobulina A
ES = error standart de las medias
Num = Numero de aves
2.3 Análisis Estadístico
Se medirá el análisis de varianza (ANOVA) para 4 estudios seleccionados con la pregunta para la hipótesis nula (H0): el uso de β-glucanos 1,3/1,6, aumenta la secreción de Inmunoglobulina A (sIgA), en respuesta a aves desafiadas con Salmonella enteritidis
μi j = Promedio de todas las respuestas de las unidades experimentales que recibieron el tratamiento i.
Ei j = Error residual de la unidad j que recibió el tratamiento i

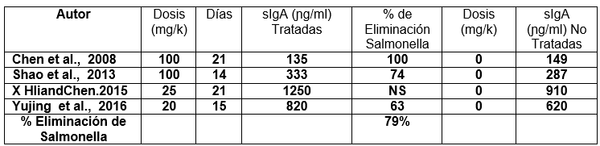
Respuesta de la Eliminación de Salmonella enteritidis con el uso β-glucanos 1,3/1,6 en pollos de engorde con uso continuo del dia1-21 de edad, Desafiados a diferentes dosis y recuperación en macerados de Hígados.
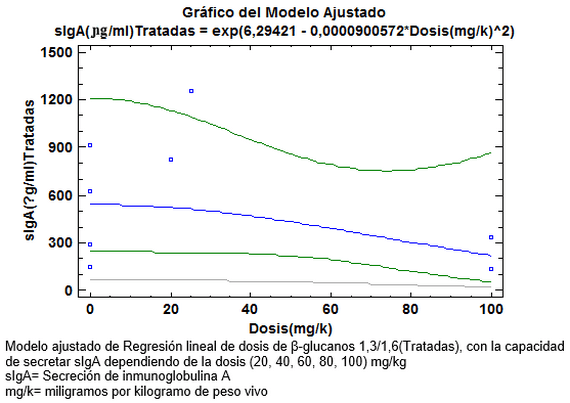
sIgA= Secreción de inmunoglobulina A
mg/k= miligramos por kilogramo de peso vivo.
4. Conclusiones
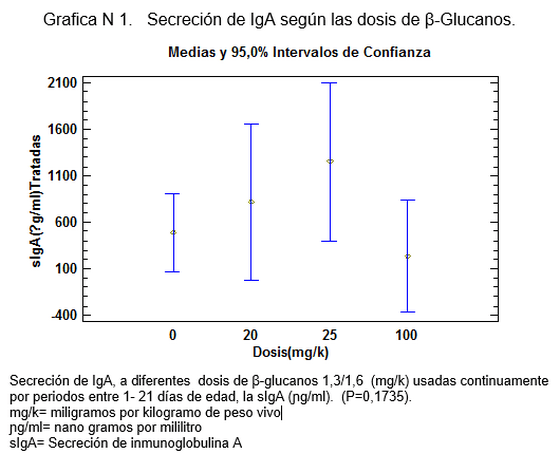
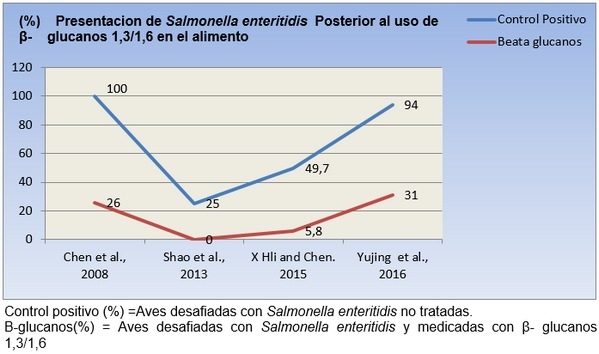
El meta-análisis es concluyente, demostrando que la sIgA en aves desafiadas con Salmonella se aumenta a partir de 20mg/kg (Ver grafica N 1) , para demostrar cual es la dosis adecuada se corre la prueba de multiples rangos analizando la variable de la gran media, cuyo criterio ratifica que los dosis medias de 20mg/k -25mg/k son las de mayor capacidad secretoria de IgA , porque están por encima del Límite Superior de las Medias al 95% (LSD > 95%) (Ver grafica N 2), De igual manera varios autores han corroborado el incremento en la secreción sIgA a diferentes dosis de β-glucanos [23,25], la confirmación de los datos anteriores nos dio las herramientas para calcular la ecuación de Regresión Lineal, para suplementar aves con β-glucanos1, 3/1,6 en pollos desafiados con Salmonella enteritidis.
[ sIgA (
Además al analizar por la prueba de Fisher a que días se detecta el incremento de la secreción de sIgA, arroja que se obtiene a los 15 días post consumo de los β-glucanos 1,3/1,6, dicha farmacocinética es demostrada por Cox et al.,2010[10].
[1] Grimont, P.A.D., Grimont, F. and Bouvet, P. (2000). Taxonomy of the genus Salmonella. In: Wray, A. and Wray, C. (eds.) Salmonella in domestic animals. CAB International, Oxford, England, p. 1-17
[2] Uzzau, S., Brown, D.J., Wallis, T., Rubino, S., Leori, G., Bernard, S., Casadesús, J., Platt, D.J. and Olsen, J.E. (2000). Host adapted serotypes of Salmonella enterica. Epidemiology and Infection, 125, 229-255.
[3] Mead, P.S., Slutsker, L., Dietz, V., McCaig, L.F., Bresee, J.S., Shapiro, C., Griffin, P.M. and Tauxe, R.V. (1999). Food-related illness and death in the United States. Emerging and Infection Diseases, 5, 607-625.
[4] Glynn, M.K., Bopp, C., Dewitt, W., Dabney, P., Mokhtar, M. and Angulo, F.J. (1998). Emergence of multidrug-resistant Salmonella enterica serotype Typhimurium DT104 infections in the United States. New England Journal of Medecine, 338, 1333- 1338.
[5] Instituto Nacional de Salud (2011). Perfil de riesgo Salmonella spp. (no tifoideas) en pollo entero y en piezas, Ministerio de protección social, PP. 68
[6] Tsukita, S., M. Furuse, and M. Itoh. (2001). Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2:285–293
[7] Catalioto, R. M., C. A. Maggi, and S. Giuliani. (2011). Intestinal epithelial barrier dysfunction in disease and possible therapeuticalI interventions. Curr. Med. Chem. 18:398–426.
[8] Ulluwishewa, D., R. C. Anderson, W. C. McNabb, P. J. Moughan, J. M. Wells, and N. C. Roy. (2011). Regulation of tight junction permeability by intestinal bacteria and dietary components. J.Nutr. 141:769–776.
[9] Griffin, A. J., and S. J. McSorley. (2011). Development of protective immunity to Salmonella, a mucosal pathogen with a systemic agenda. Mucosal Immunol. 4:371–382.
[10] Vandeplas, S., R. Dubois Dauphin, Y. Beckers, P. Thonart, and A. Thewis. (2010). Salmonella in chicken: Current and developing strategies to reduce contamination at farm level. J. Food Prot. 73:774–785.
[11] Manners, D. J., A. J. Masson, J. C. Patterson, H. Bjorndal, and B. Lindbergh. (1973). The structure of a beta-(1–6)-d-glucan from yeast cell walls. Biochem. J. 135:31–36
[12] Soltanian, S., E. Stuyven, E. Cox, P. Sorgeloos, and P. Bossier. (2009). Beta-glucans as immunostimulant in vertebrates and invertebrates. Crit. Rev. Microbiol. 35:109–138.
[13] Volman, J. J., J. D. Ramakers, and J. Plat. (2008). Dietary modulation of immune function by beta-glucans. Physiol. Behav.94:276–284
[14] Cheung, N.-K., S. Modak, A. Vickers, and B. Knuckles. (2002). Orally administered ß-glucans enhance anti-tumor effectsof monoclonal antibodies. Cancer Immunol. Immunother.51:557–564.
[15] Lowry, V. K., M. B. Farnell, P. J. Ferro, C. L. Swaggerty, A. Bahl, and M. H. Kogut. 2005. Purified ß-glucan as an abiotic feed additive up-regulates the innate immune responsein immature chickens against Salmonella entérica serovar Enteritidis. Int. J. Food Microbiol. 98:309–318.
[16] M.A.D. Saleh, H.A.T. Grecco, D.A. Berto, C.R. Padovani, R.O. Orsi, M.L.P. Tse, (2015) Int.J. Biol. Macromol. 80. 659–667.
[17] Guo, Y., R. A. Ali, and M. A. Qureshi. (2003). The influence of ß-glucan on immune responses in broiler chicks. Immupharmacol. Immunotoxicol. 3:461–472.
[18] Chae, B. J., J. D. Lohakare, W. K. Moon, S. L. Lee, Y. H. Park, and T.-W. Hahn. (2006). Effect of supplementation of ß-glucan on the growth performance and immunity in broilers. Res. Vet. Sci. 80:291–298.
[19] Cox, C. M., C. H. Stuard, S. Kim, A. P. McElroy, M. R. Bedford, and R. A. Dalloul. (2010ª). Immune responses to dietary beta glucan in broiler chicks during an Eimeria challenge. Poult. Sci.89:2597–260.
[20] Huff, G. R., W. E. Huff, N. C. Rath, and G. Tellez. 2006. Limited treatment with beta-1,3/1,6-glucan improves production values of broiler chickens challenged with Escherichia coli. Poult. Sci. 85:613–618.
[21] Ducoing A.M (2016) Diseño estadístico de experimentos. Estadística para veterinarios y zootecnistas NEWTON.PP 207
[22] Mantis, N. J., N. Rol, and B. Corthesy. (2011). Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucos Immunol. 4:603–611.
[23] Revolledo, L., C. S. Ferreira, and A. J. Ferreira.( 2009). Prevention of Salmonella Typhimurium colonization and organ invasion by combination treatment in broiler chicks. Poult. Sci. 88:734–743.
[24] Guo, Y., R. A. Ali, and M. A. Qureshi. (2003). The influence of beta glucan on immune responses in broiler chicks. Immunopharma col Immunotoxicol. 25:461–472.
[25] Y. Shao, Y. Guo, Z. Wang (2013), Yeast D Glucan induced microbial peptide Expression against Salmonella infection Poult. Sci. 92 1764–1773
[26] Berg, R. D. 1995. Bacterial translocation from the gastrointestinal tract. Trends Microbiol. 3:149–154.
[27] Remus. A, L Hatchild, I. Andretta, M. Kipper (2014) A Meta Análisis of the feed intake and grow performance of broiler chikens challenged of bacterials. Poultry Sáciense 93:1149-1158











