Producción in vitro de embriones a partir de complejos cúmulos oocitos tipo II en bovinos Bos indicus
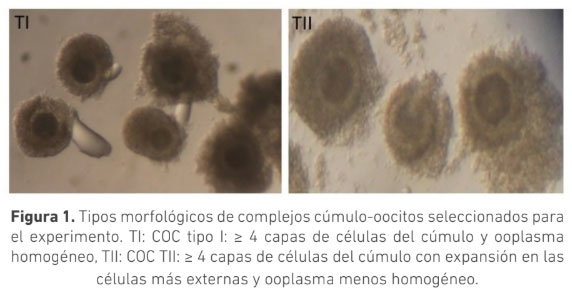
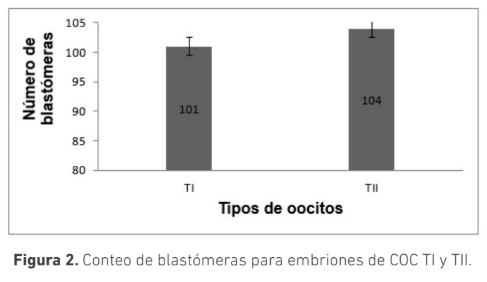
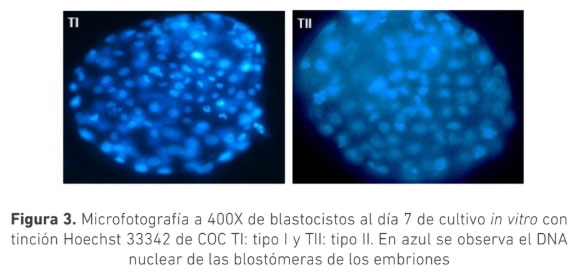
La selección morfológica de los complejos cúmulo-oocito (COC) es crucial para la producción de embriones in vitro. Se ha sugerido que COC que muestran signos de atresia poseen capacidad de generar embriones. El objetivo de este trabajo fue evaluar el efecto de la morfología de los COC provenientes de animales Bos indicus con signos de atresia temprana y sin signos de atresia sobre la producción de embriones in vitro. Se clasificaron COC obtenidos de ovarios de faenado en dos grupos: Tipo I (TI): ooplasma homogéneo con ≥ 4 capas de células del cumulo (CC) compactas y Tipo II (TII): ooplasma granular y ≥ 4 capas de CC ligeramente expandidas. Los COC fueron madurados por 24 horas en medio TCM199 y posteriormente fueron fertilizados in vitro durante 18 h. Los presuntos cigotos se cultivaron in vitro por siete días en medio SOF modificado. La calidad embrionaria se determinó por conteo de blastómeras posterior a tinción con Hoechst 33342. Se usó la prueba t para determinar diferencias estadísticas. La tasa de clivaje para los COC, TI (n=220) y TII (n=161), fue 88 ± 4 % y 89 ± 8 % respectivamente (p>0,05); la tasa de desarrollo embrionario fue 36 ± 7 % y 33 ± 8 % (p>0,05) respectivamente. El conteo de blastómeras para ambos grupos fue de (TI:101,TII:104) (n=10), (p>0,05). Los resultados de este trabajo permiten concluir que no hay diferencia en la cantidad y calidad de embriones producidos in vitro utilizando COC tipo I o tipo II, sugiriendo que ambas calidades podrían ser usadas en la producción de embriones in vitro a partir de animales Bos indicus.
Palabras clave: atresia, competencia, complejo cumulo-oocito.

1. Perry G. Statistics of embryo collection and transfer in domestic farm animals. Embryo Transfer Newsletter. 2014; 32: 14-26. http://www.iets.org/pdf/comm_ data/December2015.pdf
2. Thibier M. A contrasted year for the world activity of the animal embryo transfer industry: a report from the IETS data retrieval committee. IETS newsletter. 2002; 20 (4): 13-19.
3. Perry g. 2012 statistics of embryo collection and transfer in domestic farm animals. 2013. http://www.iets.org/pdf/comm_data/December2013.pdf
4. Lonergan P, Rizos D, Gutierrez-Adan A, Fair T, Boland MP. Oocyte and embryo quality: effect of origin, culture conditions and gene expression patterns. Reproduction in Domestic Animals. 2003; 38 (4): 259-267. https://www.ncbi.nlm.nih.gov/pubmed/12887565
5. Rizos D, Lonergan P, Boland MP, et al. Analysis of Differential Messenger RNA Expression Between Bovine Blastocysts Produced in Different Culture Systems: Implications for Blastocyst Quality 1. Biology of reproduction. 2002; 66 (3): 589-595. https://academic.oup.com/biolreprod/article/66/3/589/2723791/Analysis
6. Rizos D, Ward F, Duffy P, Boland MP, Lonergan P. Consequences of bovine oocyte maturation, fertilization or early embryo evelopment in vitro versus in vivo: implications for blastocyst yield and blastocyst quality. Molecular reproduction and development. 2002; 61 (2): 234-248. https://www.ncbi.nlm.nih.gov/pub-med/11803560
7. Rizos D, Gutierrez-Adan A, Perez-Garnelo S, De La Fuente J, Boland MP, Lonergan P. Bovine Embryo Culture in the Presence or Absence of Serum: Implications for Blastocyst Development, Cryotolerance, and Messenger RNA Expression 1. Biology of reproduction. 2003; 68 (1):236-243. https://www.ncbi.nlm.nih.gov/pubmed/12493719
8. Havlicek V, Lopatarova M, Cech S, et al. In vivo culture of bovine embryos and quality assessment of in vivo vs. in vitro produced embryos. Vet Med–Czech. 2005; 50 (4):149-157. http://vri.cz/docs/vetmed/50-4-149.pdf
9. Warzych E, Pers-Kamczyc E, Krzywak A, Dudzinska S, Lechniak D. Apoptotic index within cumulus cells is a questionable marker of meiotic competence of bovine oocytes matured in vitro. Reproductive biology. 2013; 13 (1):82-87. https://www.ncbi.nlm.nih.gov/pubmed/23522075
10. Mapletoft RJ, Hasler JF. Assisted reproductive technologies in cattle: a review. Revue Scientifique et Technique-Office International des Epizooties. 2005; 24 (1):393. http://vet.hcmuaf.edu.vn/data/file/application20review.pdf
11. Iager AE, Kocabas AM, Otu HH, et al. A novel biomarker signature expressed in human cumulus cells predicts oocyte pregnancy potential during Art. Human Reproduction. 2012; 27: ii1-ii3. https://www.researchgate.net/publica-tion/293568921_A_novel_biomarker_signature_expressed_in_human_cumu-lus_cells_predicts_oocyte_pregnancy_potential_during_ART?ev=prf_high
12. De Loos F, Van Vliet C, van Maurik Pv, Kruip TAM. Morphology of immature bovine oocytes. Gamete research. 1989; 24 (2): 197-204. https://www.ncbi.nlm.nih.gov/pubmed/2793058
13. de Wit AA, Wurth YA, Kruip TA. Effect of ovarian phase and follicle quality on morphology and developmental capacity of the bovine cumulus-oocyte complex. J Anim Sci. 2000; 78 (5): 1277-1283. https://dl.sciencesocieties.org/publications/jas/abstracts/78/5/1277?access=0&view=pdf
14. Kastrop PMM, Bevers MM, Destree OHJ, Kruip TAM. Analysis of protein synthesis in morphologically classified bovine follicular oocytes before and after maturation in vitro. Molecular reproduction and development. 1990; 26 (3): 222-226. https://www.ncbi.nlm.nih.gov/pubmed/2375875
15. Van Soom A, Tanghe S, De Pauw I, Maes D, de Kruif A. Function of the cumulus oophorus before and during mammalian fertilization. Reprod Domest Anim. 2002; 37 (3): 144-151. https://www.ncbi.nlm.nih.gov/pubmed/12071888
16. de Wit AAC, Kruip TAM. Bovine cumulus-oocyte-complex-quality is reflected in sensitivity for α-amanitin, oocyte-diameter and developmental capacity. Animal Reproduction Science. 2001; 65 (1–2): 51-65. https://www.ncbi.nlm.nih.gov/pub-med/11182508
17. Bilodeau-Goeseels S, Panich P. Effects of oocyte quality on development and transcriptional activity in early bovine embryos. Anim Reprod Sci. 2002; 71 (3-4): 143-155. https://www.ncbi.nlm.nih.gov/pubmed/12047924
18. Urrego R, Herrera-Puerta E, Chavarria NA, Camargo O, Wrenzycki C, Rodriguez-Osorio N. Follicular progesterone concentrations and messenger RNA expression of MATER and OCT-4 in immature bovine oocytes as predictors of developmental competence. Theriogenology. 2015; 83 (7): 1179-1187. https://www.ncbi.nlm.nih.gov/pubmed/25662108
19. Kussano NR, Leme LO, Guimarães ALS, Franco MM, Dode MAN. Molecular markers for oocyte competence in bovine cumulus cells. Theriogenology. 2015. https://www.ncbi.nlm.nih.gov/pubmed/26792377
20. Velez IC, Ramirez MM, Chica AI, et al. 150 proteome of bovine cumulus cells as related to oocyte morphology and in vitro embryo production. Reproduction, Fertility and Development. 2017; 29 (1): 183-183. https://www.researchgate.net/publication/312009043_150_PROTEOME_OF_BOVINE_CUMULUS_CELLS_AS_RE-LATED_TO_OOCYTE_MORPHOLOGY_AND_IN_VITRO_EMBRYO_PRODUCTION
21. Li HJ, Liu DJ, Cang M, et al. Early apoptosis is associated with improved developmental potential in bovine oocytes. Anim Reprod Sci. 2009; 114 (1-3): 89-98. https://www.ncbi.nlm.nih.gov/pubmed/19008057
22. Urrego R, Tarazona A, Olivera Ángel M, Camargo O. Simplificación de la fertilización de ovocitos durante la producción in vitro de embriones bovinos. Revista Colombiana de Ciencias Pecuarias. 2008; 21 (3): 398-405. http://www.scielo.org.co/scielo.php?script=sci_abstract&pid=S0120-06902008000300009&lng=es&nr-m=iso
23. Gordon I, Lu KH. Production of embryos in vitro and its-impact on livestock production. Theriogenology. 1990; 33 (1): 77-87. http://www.theriojournal.com/article/0093
24. Haraguchi T, Ding D-Q, Yamamoto A, Kaneda T, Koujin T, Hiraoka Y. Multiple-color fluorescene imaging of chromosomes and microtubules in living cells. Cell structure and function. 2001; 24 (5): 291-298. https://www.ncbi.nlm.nih.gov/pubmed/15216885
25. Bernal-Ulloa SM, Heinzmann J, Herrmann D, et al. Cyclic AMP affects oocyte maturation and embryo development in prepubertal and adult cattle. PloS one. 2016; 11 (2): e0150264. http://journals.plos.org/plosone/article?id=10.1371/journal.pone
26. Duranthon V, Renard JP. The developmental competence of mammalian oocytes: a convenient but biologically fuzzy concept. Theriogenology. 2001; 55 (6): 1277-1289. https://www.ncbi.nlm.nih.gov/pubmed/11327684
27. Khurana NK, Niemann H. Effects of oocyte quality, oxygen tension, embryo density, cumulus cells and energy substrates on cleavage and morula/blastocyst formation of bovine embryos. Theriogenology. 2000; 54 (5): 741-756. https://www.ncbi.nlm.nih.gov/pubmed/11101035
28. Blondin P, Sirard M-A. Oocyte and follicular morphology as determining characteristics for developmental competence in bovine oocytes. Molecular Reproduction and Development. 1995; 41 (1): 54-62. https://www.ncbi.nlm.nih.gov/pub-med/7619506
29. Urrego R, Bernal-Ulloa SM, Chavarria NA, et al. Satellite DNA methylation status and expression of selected genes in Bos indicus blastocysts produced in vivo and in vitro. Zygote. 2017: 1-10. https://www.ncbi.nlm.nih.gov/pubmed/28137339
30. Bunel A, Jorssen EP, Merckx E, Leroy JL, Bols PE, Sirard MA. Individual bovine in vitro embryo production and cumulus cell transcriptomic analysis to distinguish cumulus-oocyte complexes with high or low developmental potential. Theriogenology. 2015; 83 (2): 228-237. https://www.ncbi.nlm.nih.gov/pubmed/25442391
31. Baldoceda-Baldeon LM, Gagné D, Vigneault C, Blondin P, Robert C. Improvement of bovine in vitro embryo production by vitamin K2 supplementation. Reproduction. 2014; 148 (5): 489-497. https://www.ncbi.nlm.nih.gov/pubmed/25161289
32. Aziz NAA, Osman NA, Bidin H, Embong WK, Hashim NH. Influence of Early Apoptosis Incidence on In Vitro Maturation of Bovine Oocytes. APCBEE Procedia. 2014; 8: 272-276. http://www.sciencedirect.com/science/article/pii/S2212670814001195
33. Borup R, Thuesen LL, Andersen CY, et al. Competence classification of cumulus and granulosa Cell transcriptome in embryos matched by morphology and female age. PloS one. 2016; 11 (4): e0153562. http://journals.plos.org/plosone/article?id=10.1371/journal.pone
34. Nivet A-L, Vigneault C, Blondin P, Sirard M-A. Changes in granulosa cells’ gene expression associated with increased oocyte competence in bovine. Reproduction. 2013; 145 (6): 555-565. https://www.ncbi.nlm.nih.gov/pubmed/23564726
35. Bao Z-J, Zhao S, Haq IU, Zeng S-M. Recombinant bovine interferon-τ enhances in vitro development of bovine embryos by upregulating expression of connexin 43 and E-cadherin. Journal of dairy science. 2014; 97 (11): 6917-6925. https://www.ncbi.nlm.nih.gov/pubmed/25242422
36. Santana PPB, Carvalho CMF, da Costa NN, et al. Effect of dexamethasone on development of in vitro–produced bovine embryos. Theriogenology. 2014; 82 (1):10-16. https://www.ncbi.nlm.nih.gov/pubmed/24656431









