Inhibir la proliferación del virus de PRRS en cerdos
Actividad Antiviral de la Tylvalosina Tartrato Sobre el Virus de PRRSV Por la Vía de la Activación Nrf2-HMOX1
El síndrome respiratorio y reproductivo porcino (PRRS) es una de las enfermedades infecciosas de los cerdos de mayor importancia económica en el mundo, Es la causante de una fuerte inflamación y una inmunosupresión muy marcada. Se han implementado diversas estrategias para su control , sin embargo su alta diversidad genética y la dificultad para entender como evade a el sistema inmune para causar abortos y una fuerte enfermedad respiratoria complicada en confección con otros patógenos; es de vital importancia desarrollar nuevas estrategias para combatir la infección, en esta revisión sistemática sobre la capacidad antiviral de la Tylvalosina tartrato, macrolido semisintetico de tercera generación(3-A 4-O Acetyl Isovaleril Tartrato de Tylosina) los datos de esta investigación demuestran que este antibiótico puede reducir la cantidad del virus dependiendo de la concentración. por que se reduce la oxidación celular generada por los radicales libres(ROS) al disminuir la expresión molecular de la enzima Hemo Oxigenasa 1 (HMOX 1) demostrando que la TV produce un efecto antinflamatorio y antiviral frente al virus de PRRS, reportando como la dosis mas efectiva es de 40 mg/ml la cual disminuye la carga viral en un 49.6%
Palabras claves: Síndrome respiratorio y reproductivo porcino(PRRS), Tylvalosina (TV), Radicales libres dependientes del oxigeno (ROS), Lipopolisacaridos(LPS), Factores de transcripción (NF-κB).
Abstract
Porcine reproductive and respiratory syndrome (PRRS) is one of the most economically important infectious diseases of pigs worldwide. It is the cause of strog inflammation and very marked inmmunosuppresion. various management measures have been implemented to control PRRS, However, due to high genetic diversity and insufficient understanding of the pathogenesis and immunological mechanisms to understand how immune evade to cause abortions and severe respiratory complicated by other pathogens; is the vitality important to develop new strategies to combact infection. In this systematic review on the antiviral capacity of Tylvalosin tartrate a third-generation semyshithetic macrolide (3-A 4-O Acetyl Isovaleryl Tylosyn Tartrate) The data from this research demonstrate to this antibiotic can reduce the amount on the virus depending on the concentration because cellular oxidation generated by free radicals (ROS) is reduced by decresing the cmolecular expression of the enzyme Heme Oxygenase 1(HMOX 1) demonstrating that TV produce antinflamatory and antiviral agaist the PRRS virus,reporting that the most effective doses is the 40 mg/ml wich reduce the viral load in 49.6%.
Keywords: Porcine reproductive and respiratory syndrome (PRRS), Tylvalosin (TV), Oxygen-dependent free radicals (ROS), Lipopolysaccharides (LPS), Transcription factors (NF-κB).
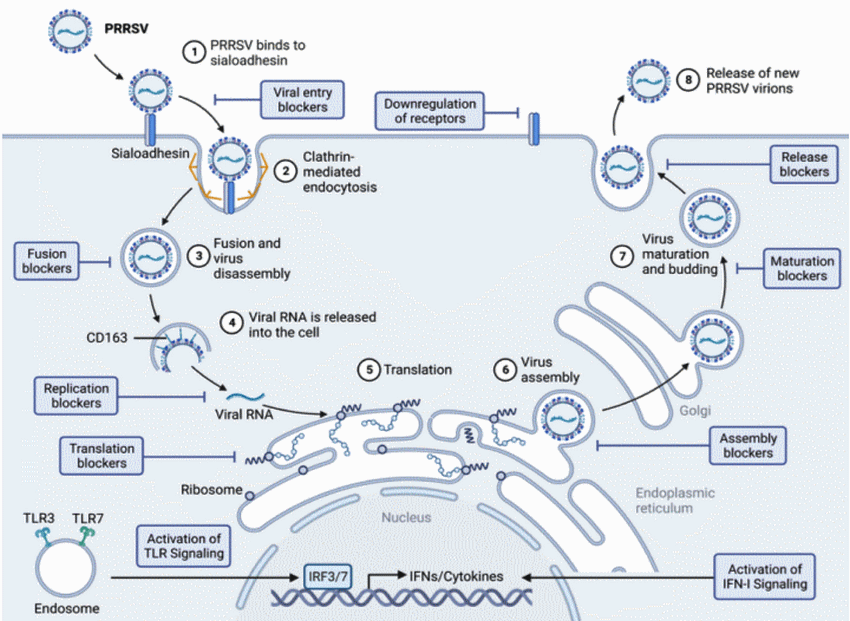
El ciclo de vida del Virus de PRRS comienza con la unión a la siloadehesina(1) que desencadena la endocitosis estimulada por la claritrina (2) para fusionarse con un endosoma (3) luego se desensambla el virus y se libera ARN viral (4) para su teaduccion en el retículo endoplasmatico (5) las proteínas traducidas se ensamblan en nuevos viriones (5,6,7) esta nueva serie de viriones se libera fuera de la célula (8), y por lo tanto el virus de PRSS nunca interactuó con el IRF3/ que bloquea la fosforilacion no activando los mensajeros de los Interferones INFs
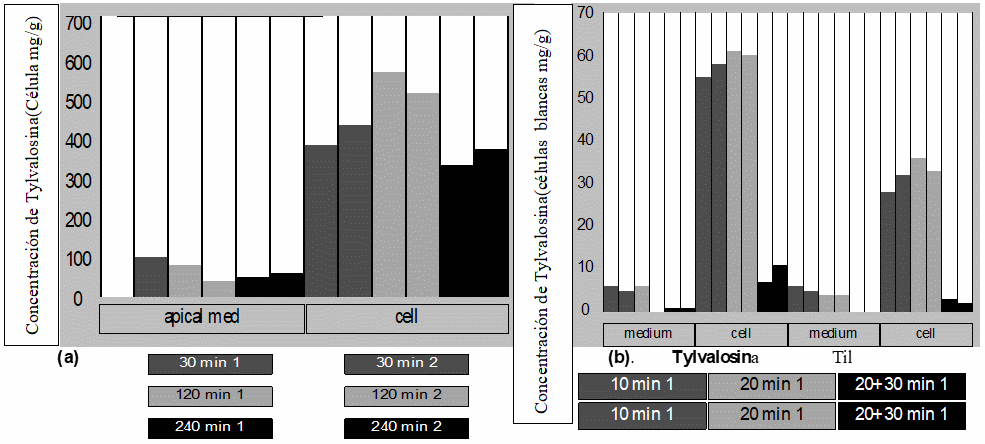
F(a) Transpaso transepitelial a celulas apicales y acumulacion intracelular en celulas Caco-2 de Tylvalosina (apical med: celulas apicales mediales, cell: Celulas entericas).Figura N.3 (b) Concentracion de Tylvalosina en celulas blancas en μg/ml en un periodo de tiempo de 10, 20, y 30 minutos a una concentración de 10μg/ml, Adaptado de Stuart.A.et al; 2007[20]).
2.0 Materiales y Metodos
2.1 Revision de literatura
2.2 Búsqueda de literatura
Se uso la combinación de las palabras claves de la pregunta primaria que fueron las siguientes:
[(Pig OR Swine OR Herd)AND(Antiviral Effect Against to Porcine Reproductive and Respiratory Syndrome OR Prrs )AND(Tylvalosin OR 3-A, 4-O Acetyl Tylosin Tartrate)],Se identificaron 6 estudios en ingles, que contienen la información del efecto antiviral de la TV frente a desafíos del virus de PRRS, comparados con el grupo control negativo, se seleccionaron 5 estudios y se elimino 1 por no contener información sobre el porcentaje (%) de disminucion del virus de PRRS
2.3 Análisis estadístico
Cuadro N1. Estudios seleccionados (n=5) que cumplen con los requisitos para la evaluación del efecto antiviral de la Tylvalosina sobre el virus del síndrome respiratorio y reproductivo porcino.
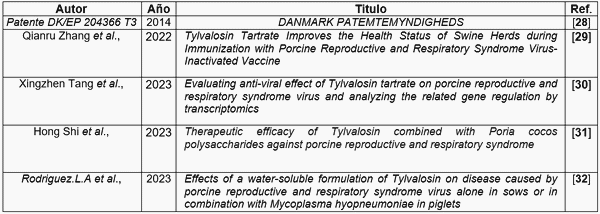
Patente DK/EP 204366 T3: Patente autorizada del gobierno de Dinamarca en 2014, sobre ensayos de actividad antiviral de la Tylvalosina.
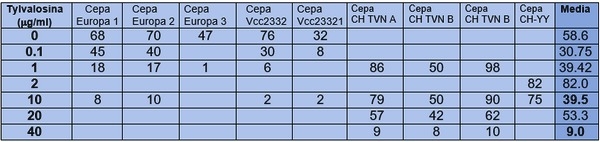
(µg/ml): Dosis de Tylvalosina en microgramos/mililitros, Cepa: Cepas de el Virus de PRRS evaluadas no homogeneas, Dosis 0: Dosis 0 del grupo control negativo, Media: Media geometrica según la dosis usada
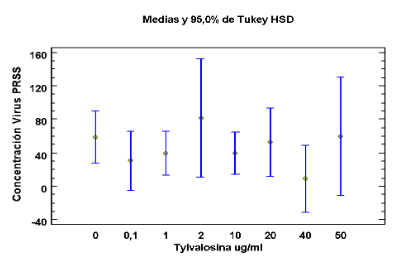
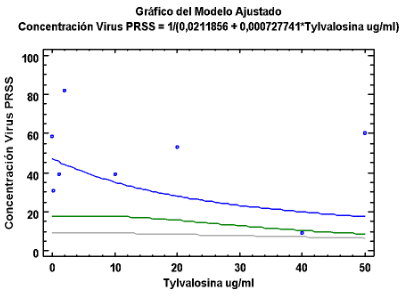
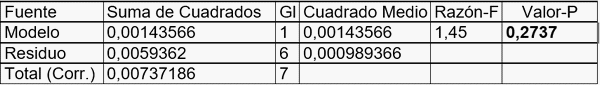
Coeficiente de Correlación = 0,441304
R-cuadrada = 19,4749 %
R-cuadrado (ajustado para g.l.) = 6,05407 %
Error estándar del est. = 0,0314542
Error absoluto medio = 0,0188995
Estadístico Durbin-Watson = 1,96386 (P=0,3055)
Autocorrelación de residuos en retraso 1 = -0,294889
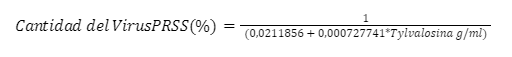
[1] Wensvoort, G., Terpstra, C., Pol, J. M., Laak, E. A., Bloemraad, M., de Kluyver, E. P., et al. (1991). Mystery swine disease in The Netherlands: the isolation of Lelystad virus. Vet. Q. 13, 121–130. doi: 10.1080/01652176. 1991.9694296
[2] Morrison, R. B., Collins, J. E., Harris, L., Christianson, W. T., Benfield, D. A., Chladek, D. W., et al. (1992). Serologic evidence incriminating a recently isolated virus (ATCC VR-2332) as the cause of swine infertility and respiratory syndrome (SIRS). J. Vet. Diagn. Investig. 4, 186–188. doi: 10.1177/ 104063879200400212
[3] Carstens, E. B. (2010). Ratification vote on taxonomic proposals to the international committee on taxonomy of viruses. Arch. Virol. 155, 133–146. doi: 10.1007/s00705-009-0547-x
[4] Kappes, M. A., and Faaberg, K. S. (2015). PRRSV structure, replication and recombination: origin of phenotype and genotype diversity. Virology 479, 475–486.doi: 10.1016/j.virol.2015.02.012
[5] Lunney, J. K., Fang, Y., Ladinig, A., Chen, N., Li, Y., Rowland, B., et al. (2016). Porcine reproductive and respiratory syndrome virus (PRRSV): pathogenesis and interaction with the immune system. Annu Rev Anim Biosci. 4, 129–154. doi: 10.1146/annurev-animal-022114-111025.
[6] Jonas, S., and Izaurralde, E. (2015). Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 16, 421–433. doi: 10.1038/nrg3965 .
[7] Xiao, S., Wang, X., Ni, H., Li, N., Zhang, A., Liu, H., et al. (2015). MicroRNA miR-24-3p promotes porcine reproductive and respiratory syndrome virus replication through suppression of heme oxygenase-1 expression. J. Virol. 89, 4494–4503. doi: 10.1128/JVI.02810-14.
[8] Zhang, Q., Huang, C., Yang, Q., Gao, L., Liu, H. C., Tang, J., et al. (2016). MicroRNA-30c modulates type I IFN responses to facilitate porcine reproductive and respiratory syndrome virus infection by targeting JAK1. J. Immunol. 196, 2272–2282. doi: 10.4049/jimmunol.1502006.
[9] Wang G, Li L, Yu Y, Tu Y, Tong J, Zhang C, et al. (2016) Highly pathogenic porcine reproductive and respiratory syndrome virus infection and induction of apoptosis in bone marrow cells of infected piglets. J Gen Virol. 97:1356–61. doi: 10.1099/ jgv.0.000454.
[10] Amarilla SP, Gómez-Laguna J, Carrasco L, Rodríguez-Gómez IM, Caridad YOJM, Graham SP, et al. (2017) Porcine reproductive and respiratory syndrome type 1 viruses induce hypoplasia of erythroid cells and myeloid cell hyperplasia in the bone marrow of experimentally infected piglets independently of the viral load and virulence. Vet Microbiol. 201:126–35. doi: 10.1016/j.vetmic.2016.12.040.
[11] Calzada-Nova G, Schnitzlein WM, Husmann RJ, Zuckermann FA. (2011) North American porcine reproductive and respiratory syndrome viruses inhibit type I interferon production by plasmacytoid dendritic cells. J Virol. 85:2703–13. doi: 10.1128/jvi.01616-10.
[12] Albina E, Piriou L, Hutet E, Cariolet R, L'Hospitalier R. Immune responses in pigs infected with porcine reproductive and respiratory syndrome virus (PRRSV). (1998) Vet Immunol Immunopathol. 61:49–66. doi: 10.1016/s0165-2427(97)00134-7.
[13] Ivashkiv, L. B., and Donlin, L. T. (2014). Regulation of IFN-I responses. Nat. Rev. Immunol. 14, 36–49. doi: 10.1038/nri3581.
[14] Garcia-Sastre, A., and Biron, C. A. (2006). Type 1 interferons and the virus-host relationship: a lesson in detente. Science 312, 879–882. doi: 10.1126/science.1125676.
[15] Sagong, M., and Lee, C. (2011). Porcine reproductive and respiratory syndrome virus nucleocapsid protein modulates interferon-beta production by inhibiting IRF3 activation in immortalized porcine alveolar macrophages. Arch. Virol. 156, 2187–2195. doi: 10.1007/s00705-011-1116-7.
[16] Zhao, K., Li, L. W., Jiang, Y. F., Gao, F., Zhang, Y. J., Zhao, W. Y., et al. (2019). Nucleocapsid protein of porcine reproductive and respiratory syndrome virus antagonizes the antiviral activity of TRIM25 by interfering with TRIM25-mediated RIG-I ubiquitination. Vet. Microbiol. 233, 140–146. doi: 10.1016/j.vetmic.2019.05.003.
[17] Zhang X and Feng W-H (2021) Porcine Reproductive and Respiratory Syndrome Virus Evades Antiviral Innate Immunity via MicroRNAs Regulation. Front. Microbiol. 12:804264. doi: 10.3389/fmicb.2021.804264.
[18] Fredmoore L. Orosco (2024). From Nature's Pharmacy to Swine Health: Harnessing Natural Compounds against PRRSV Infection Slov Vet Res . Vol 61 No 1 doi: 10.26873/SVR-1789-2023.
[19] Parvathaneni V, Kulkarni NS, Muth A, Gupta V. (2019)Drug repurposing: a promising tool to accelerate the drug discovery process. Drug Discov Today24:2076–85. doi: 10.1016/j.drudis.2019.06.014.
[20] Stuart AD, Brown TDK, Imrie G, Tasker JB, Mockett APA. (2007)Intra-cellular accumulation and trans-epithelial transport of aivlosin, tylosin and tilmicosin. Pig J.60:26–35.
[21] Du Y, Yoo D, Paradis MA, Scherba G. (2011)Antiviral activity of tilmicosin for type 1 and type 2 porcine reproductive and respiratory syndrome virus in cultured porcine alveolar macrophages. Antivir Antiretrovir. 3:28–33. doi: 10.4172/jaa.1000031.
[22] Zhao Z, Tang X, Zhao X, Zhang M, Zhang W, Hou S, et al. (2014) Tylvalosin exhibits anti-inflammatory property and attenuates acute lung injury in different models possibly through suppression of NF-kappaB activation. Biochem Pharmacol. 90:73–87. doi: 10.1016/j.bcp.2014.04.015.
[23] Guedes, R.M.C.; França, S.A.; Machado, G.S.; Blumer, M.A.; da Costa Cruz Jr, E.C. (2009)Use of tylvalosin‐medicated feed to control porcine proliferative enteropathy. Vet. Rec., 165, 342–345.
[24] Hernandis, V.; Escudero, E.; Galecio, J.S.; Marín, P. (2022).Quantification and Determination of Stability of Tylvalosin in Pig Plasma by Ultra‐High Liquid Chromatography with Ultraviolet Detection. Animals, 12, 1385. https://doi.org/10.3390/ani12111385
[25] Elbadawy, M.; Aboubakr, M.; Abugomaa, A. . (2019) Pharmacokinetics of tylvalosin in broiler Turkeys (Meleagris gallopavo) after sin‐ gle intravenous and oral administration. Front. Vet. Sci. 2019, 6, 6–11. https://doi.org/10.3389/fvets.2019.00355.
[26] Scorneaux, B., and Shryock,T.R. (1999).The determination of the cellular volume of avian, porcine and bovine phagocytes and bovine mammary epithelial cells and its relationship to uptake of tilmicosin. Journal ofVeterinary Pharmacology andTherapeutics,22,6–12.
[27] Stuart,L.M., and Ezekowitz, R.A.(2005). Phagocytosis: elegant complexity.Immunity,22, 539–550.
[28] Patent DK/EP 204366 T3. (2014). DANAMRK PATEMTEMYNDIGHEDS
[29] Zhang, Q.; Cui, C.; Zhang, S.; Deng, X.; Cai, X.; Wang, G. Tylvalosin Tartrate Improves the Health Status of Swine Herds during Immunization with Porcine Reproductive and Respiratory Syndrome Virus-Inactivated Vaccine. Vet.Sci.2023,10,12. https:// doi.org/10.3390/vetsci10010012
[30] Xingzhen Tang.; Cong Wang.;Weifeng Sun.; Weixin Wu.; Shaohui Sun.; Jin Wan.; Guangshan Zhu.; Nini Ma.; Xiaoping Ma.; Ruihua Xu.; Qiushi Yang.; Yindi Dai and Lei Zhou.(2023). Evaluating anti-viral effect of Tylvalosin tartrate on porcine reproductive and respiratory syndrome virus and analyzing the related gene regulation by transcriptomics. Virology Journal 20:79 https://doi.org/10.1186/s12985-023-02043-w
[31] Shi H, Luo W, Wang S, Dai J, Chen C, Li S, Liu J, Zhang W, Huang Q and Zhou R (2023) Therapeutic efficacy of tylvalosin combined with Poria cocos polysaccharides against porcine reproductive and respiratory syndrome. Front. Vet. Sci. 10:1242146.doi: 10.3389/fvets.2023.1242146
[32] Alfonso Lopez Rodriguez.; Veronica L.; Fowler Michael Huether.; David Reddick.; Christine Tait‐Burkard.; Marie O’Shea.; Stephanie Perkins, Nirosh Dias, Robin Buterbaugh and Hafid A. BenchaouI (2023). Effects of a water-soluble formulation of Tylvalosin on disease caused by porcine reproductive and respiratory syndrome virus alone in sows or in combination with Mycoplasma hyopneumoniae in piglets BMC Veterinary Research 19:31 https://doi.org/10.1186/s12917-023-03571-x
[33] Moges, R. (2017). The Immune Modulatory Effects of Tylvalosin in Porcine Neutrophils and Macrophages in vitro). University of Calgary, Calgary, AB. doi:10.11575/PRISM/28175 http://hdl.handle.net/11023/3941 .



Efectivamente, como usted señala, los antibióticos incluidos en los estudios analizados no presentan un efecto antiviral directo evidenciado sobre la replicación viral, sino más bien una posible modulación de la respuesta inmune e inflamatoria del hospedero, lo cual podría explicar los efectos beneficiosos observados. Coincidimos plenamente en que esto abre una línea interesante para futuros estudios enfocados en compuestos con acción antiinflamatoria específica, que permitan dilucidar mejor este mecanismo de acción.
Respecto a las variables experimentales de cada estudio incluido en el meta-análisis, como el uso de inmunógenos en Zhang (2022) o la combinación con polisacáridos en Shi (2023), es una observación muy pertinente. Estos factores, sin duda, introducen heterogeneidad clínica y metodológica que puede influir en la estimación del efecto atribuible exclusivamente al antibiótico. En nuestro análisis se reconocen estas diferencias y se discuten sus implicaciones en la sección de limitaciones. No obstante, dado el diseño de los estudios originales, no siempre es posible disociar completamente el efecto del antibiótico de los coadyuvantes utilizados.
Consideramos que futuras investigaciones deberían contemplar diseños experimentales más controlados, que permitan evaluar de forma aislada y comparativa estos componentes, para una interpretación más precisa del efecto del antibiótico en contextos virales.
Agradecemos nuevamente su aporte al análisis crítico del estudio.

Apreciados colegas, gracias por este meta análisis, a su vez entiendo que no hay efecto directo antiviral del antibiótico conducente a disminuir la replicación viral, todo indica que hay un efecto en atenuar la respuesta inflamatoria y en este punto, sería interesante ver estudios de terapéuticos propiamente antiinflamatorios.
Por otro lado, existen variables importantes de cada estudio utilizado para el meta análisis, por ejemplo, en el Zhang (2022), hacen uso de un inmunógeno, en el de Shi (2023) usan el antibiótico combinado con polisacáridos, ¿Estos aspectos pueden conllevar a desvirtuar la interpretación de efecto antibiótico?.






.jpg&w=3840&q=75)










